The Origin of Niobium
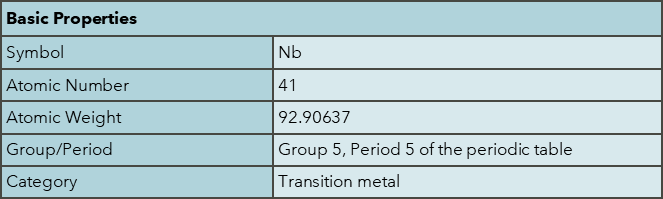
Niobium was discovered by Charles Hatchett in 1801. It is named after Niobe, the daughter of Tantalus in Greek mythology, reflecting its similarities to tantalum. Niobium is found in minerals such as pyrochlore and columbite. Major producers include Brazil (91%) and Canada (8%), with worldwide production in 2023 at 83,000 tonnes, with reserves estimated at >17,000,000 tonnes. The abundance of niobium in the continental crust is approximately 8 ppm. Niobium has high economic importance as a key enabler for green technology and acts in many ways to provide functionality to many materials critical to the sustainability agenda.